This morning, the U.S. Food and Drug Administration (FDA) published detailed data regarding 48 seafood entry line refusals in February, of which ten (20.8%) were for shrimp for reasons related to banned antibiotics.
As indicated in the table below, the FDA has already refused a total of sixteen (16) entry lines of shrimp for banned antibiotics in 2024.
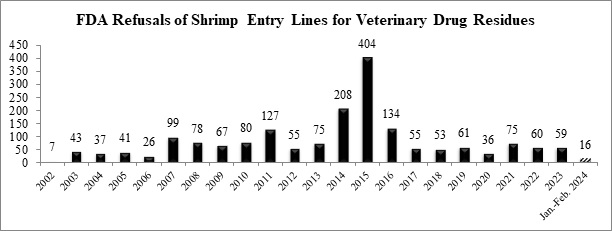
The ten (10) entry lines of shrimp refused for banned antibiotics in February were for shipments of shrimp from six exporters in four different countries (India, Japan, Thailand, and Vietnam). Of these six processors and exporters, five currently operate under a four-star Best Aquaculture Practices (BAP) certification:
- Calcutta Seafoods Pvt. Ltd. (India), a company that currently operates under a four-star BAP certification for its processing plant (P10567), with additional BAP certifications for multiple shrimp farms, and that was added to Import Alert 16-129 (“Detention Without Physical Examination of Seafood Products Due to Nitrofurans”) for its shipments of shrimp on July 19, 2023, had two entry lines refused for shrimp contaminated with nitrofurans and veterinary drug residues by the Division of West Coast Imports on February 16, 2024;
- Devi Seafoods Limited (India), a company that currently operates under four-star Best Aquaculture Practices (BAP) certifications for two processing plants (Plant 1 = P10010; Plant 2 = 10011), with an additional BAP certification for a shrimp farm (“Devi Farm Group 2”), and that was added to Import Alert 16-124 (“Detention Without Physical Examination of Aquaculture Seafood Products Due to Unapproved Drugs”) for the presence of gentian violet and leucogentian violet in its shipments of shrimp on February 1, 2024, had one entry line refused for shrimp contaminated with nitrofurans and veterinary drug residues by the Division of West Coast Imports on February 16, 2024 and one entry line refused for shrimp contaminated with nitrofurans by the Division of West Coast Imports on February 23, 2024;
- Kader Exports Private Limited, Unit 04 (India), a company that currently operates under a four-star Best Aquaculture Practices (BAP) certification for its Unit 04 processing plant (P10302), with additional BAP certifications for multiple shrimp farms, and that was added to Import Alert 16-129 (“Detention Without Physical Examination of Seafood Products Due to Nitrofurans”) for its shipments of shrimp on January 22, 2024 and to Import Alert 16-124 (“Detention Without Physical Examination of Aquaculture Seafood Products Due to Unapproved Drugs”) for leucomalachite green in its shipments of shrimp on December 15, 2023, had two entry lines refused for shrimp contaminated with nitrofurans and veterinary drug residues by the Division of Northeast Imports on February 23, 2024;
- Fimex Vn (Vietnam), a company that, as SAOTA Foods Joint Stock Company (FIMEX VN) currently operates under a four-star Best Aquaculture Practices (BAP) certification for its processing plant (P10061) and that was added to Import Alert 16-127 (“Detention Without Physical Examination of Crustaceans Due to Chloramphenicol”) for its shipments of shrimp, including breaded shrimp, on January 22, 2024, had one entry line refused for shrimp contaminated with chloramphenicol by the Division of Northeast Imports on February 5, 2024 and one entry line refused for shrimp contaminated with chloramphenicol by the Division of West Coast Imports on February 28, 2024;
- Thai Union Group Public Company Limited (Thailand), a company that currently operates under a four-star Best Aquaculture Practices (BAP) certification for its Unit 04 processing plant (P10055), with additional BAP certifications for multiple shrimp farms, and that to Import Alert 16-124 (“Detention Without Physical Examination of Aquaculture Seafood Products Due to Unapproved Drugs”) for ciprofloxacin and sulfamethoxazole in its shipments of shrimp on February 27, 2024, had one entry line refused for shrimp contaminated with veterinary drug residues by the Division of West Coast Imports on February 15, 2024; and
- Tokoro Fisheries Cooperative Association (Japan), a company that is not currently listed on Import Alert 16-124 (“Detention Without Physical Examination of Aquaculture Seafood Products Due to Unapproved Drugs”), Import Alert 16-127 (“Detention Without Physical Examination of Crustaceans Due to Chloramphenicol”), or Import Alert 16-129 (“Detention Without Physical Examination of Seafood Products Due to Nitrofurans”), had one entry line refused for shrimp contaminated with veterinary drug residues and unsafe additives by the Division of West Coast Imports on February 6, 2024;
The Southern Shrimp Alliance maintains a database of the FDA’s refusals of shrimp entry lines for reasons related to veterinary drug residues that goes back to 2002. Over that time period, the refusal announced last month of a shrimp entry line from Tokoro Fisheries Cooperative Association was the first time that the agency has ever announced refused a shrimp entry line originating from Japan for reasons related to banned antibiotics.
Also this morning, the FDA announced refusals of another six entry lines of shrimp from India (3) and Thailand (3) in February for the presence of salmonella and/or filth.
