Today, the U.S. Food and Drug Administration (FDA) published detailed data regarding 71 seafood entry line refusals in July, of which none were of shrimp for reasons related to veterinary drug residues. Accordingly, last month marked the first time since September that the FDA did not report a refusal of antibiotic-contaminated shrimp.
Moreover, the FDA reported refusing only one entry line of shrimp for any reason in July. An entry line of shrimp from the Indian shrimp exporter Edhayam Frozen Foods PVT Ltd. was refused for the presence of salmonella and for being filthy by the Division of Southwest Imports on July 25th, but otherwise no other shipments of shrimp were stopped by the agency. This represents a substantial break from the FDA’s activities this year. In comparison, during the previous three months, the agency reported refusing 55 entry lines of shrimp for filth and/or for salmonella.
Although the FDA’s reporting seems to indicate a shift away from inspecting shrimp imports, the agency appears to have significantly improved its ability to pick up violative shipments of seafood once antibiotics have been detected. Specifically, after the Division of West Coast Imports refused three entry lines of croaker shipped by the Chinese exporter Shengyuan Aquatic Food Co., Ltd. on June 1, 2023 due to the presence of veterinary drug residues, the FDA reported that a different division, the Division of Northeast Imports, subsequently refused another eleven entry lines of croaker shipped by Shengyuan Aquatic Food later in June and in July. Yet another entry line of croaker shipped by Shengyuan Aquatic Food was refused by the Division of West Coast Imports on July 19 for the presence of veterinary drug residues.
In addition, the FDA also reported taking action to prohibit the importation of short-weighted seafood. Three entry lines of squid shipped by the Chinese exporter Yantai Ruihe Foods Co., Ltd. were refused by the Division of West Coast Imports on July 17th for “added bulk.”
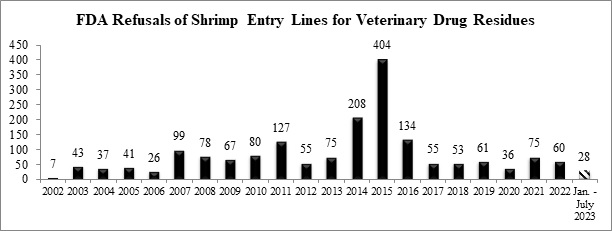
Through the first seven months of the year, the FDA has refused a total of twenty-eight entry lines of shrimp for banned antibiotics.