On Monday, the U.S. Food and Drug Administration (FDA) published detailed data regarding 44 seafood entry line refusals in August, of which just one (2.3%) was for shrimp for reasons related to banned antibiotics.
The FDA’s released data appears only to cover imports through August 24th. While only one of the reported refusals was for antibiotic-contaminated shrimp, a total of eleven (11) of the 44 refused seafood entry lines were for reasons related to antibiotics in seafood, all from China (in addition to shrimp, there were nine for croaker and one for frog).
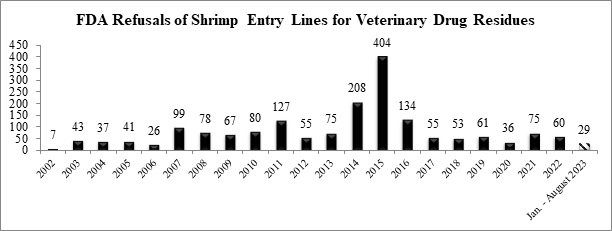
Through the first eight months of this year, the FDA has refused a total of twenty-nine entry lines of shrimp for banned antibiotics, less than half of the agency’s refusals last year.
The one entry line of shrimp refused for banned antibiotics in August was for a shipment from a Chinese shrimp exporter:
- Lianjiang Xinyang Aquatic Products Co., Ltd. (China), a company that is not on the green list of Import Alert 16-131 (“Detention Without Physical Examination of Aquacultured, Shrimp, Dace, and Eel from China – Presence of New Animal Drugs and/or Unsafe Food Additives”), had one entry line refused for shrimp contaminated with veterinary drug residues and unsafe additives by the Division of West Coast Imports on August 11, 2023.
In addition, the FDA reported the refusal of one other entry line of shrimp for the presence of salmonella in August from India’s Royale Marine Impex Pvt. Ltd., refused by the Division of Northeast Imports on August 17, 2023.
