Yesterday, the U.S. Food and Drug Administration (FDA) published detailed data regarding 23 seafood entry line refusals in September, of which four (17.4%) was for shrimp for reasons related to banned antibiotics.
The FDA’s released data appears only to cover imports through September 22nd.
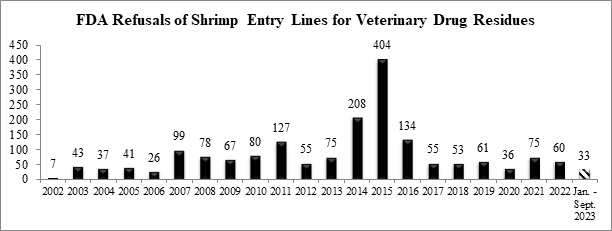
Through the three quarters of this year, the FDA has refused a total of thirty-three entry lines of shrimp for banned antibiotics.
The four entry lines of shrimp refused for banned antibiotics in September were for shipments from a single Indian shrimp exporter:
- Milesh Marine Exports Pvt. Ltd. (India), a company that was added to Import Alert 16-129 (“Detention Without Physical Examination of Seafood Products Due to Nitrofurans”) for its shipments of shrimp on June 1, 2023, had four entry lines refused for shrimp contaminated with nitrofurans and veterinary drug residues by the Division of Southwest Imports on September 18, 2023.
In addition, the FDA reported that the Division of West Coast Imports refused another three entry lines of shrimp for the presence of unsafe additives from China’s Zhangzhou Hongwei Foods Co., Ltd. on September 1, 2023.
