This morning, the U.S. Food and Drug Administration (FDA) published data reporting that there were 91 total seafood entry line refusals in December, of which seven (7.7%) were of shrimp for reasons related to banned antibiotics.
In addition, the FDA has also now reported an additional fifteen seafood entry line refusals in November, increasing the total for that month from 88 to 103. A third of these additional entry line refusals were attributed to the Indian exporter Kader Exports Private Limited, increasing the total number of entry lines of shrimp originating from that company to 50 in November. Each of the additional entry line refusals was for salmonella in Kader Exports’ shrimp. Four of the five entry line refusals were reported by the Division of Northern Border Imports on November 23rd, indicating that the FDA’s coordinated actions in response to contaminated shrimp from Kader Exports spanned four of the agency’s five import divisions (West Coast; Southeast; Northeast; and Northern Border).
The FDA’s response to shipments of contaminated shrimp from both Kader Exports Private Limited and Kader Investment & Trading Company Private Limited continued in December as well. The agency reported refusing a total of 38 more entry lines of shrimp from these companies last month, 22 for the presence of salmonella and 16 for being “filthy.” The two Indian entities, Kader Exports and Kader Investment & Trading, on their own accounted for over 45 percent of all seafood entry line refusals the last two months.
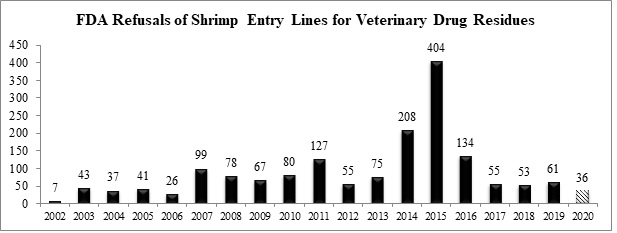
As to antibiotic contamination, with seven additional entry line refusals in December, the FDA reported a total of thirty-six (36) refusals of shrimp entry lines for reasons related to banned antibiotics in 2020. As shown in the chart below, this represents the lowest number of entry lines of shrimp refused for contamination with veterinary drug residues since 2006.
The seven shrimp entry lines refused in December for reasons related to antibiotics were for shipments from Bangladesh, China, India, and Vietnam:
- Southern Foods Limited (Bangladesh), a company that has been listed on Import Alert 16-129 (“Detention Without Physical Examination of Seafood Products Due to Nitrofurans”) since August 30, 2016 for nitrofurans in its shrimp, had four entry lines refused for shrimp contaminated with nitrofurans by the Division of Northeast Imports on December 9, 2020;
- Apex Frozen Foods Pvt Ltd (India), a company that is currently listed on Import Alert 16-129 (“Detention Without Physical Examination of Seafood Products Due to Nitrofurans”) as of December 16, 2020 for nitrofurans in its shrimp, had one entry line refused for shrimp contaminated with veterinary drug residues by the Division of West Coast Imports on December 14, 2020;
- Fuqing Yihua Aquatic Food Co., Ltd. (China), a company that is no longer on the green list of Import Alert 16-131 (“Detention Without Physical Examination of Aquacultured, Shrimp, Dace, and Eel from China – Presence of New Animal Drugs and/or Unsafe Food Additives”), had one entry line refused for breaded shrimp contaminated with veterinary drug residues and unsafe additives by the Division of West Coast Imports on December 3, 2020; and
- Seavina Joint Stock Company (Vietnam), a company that is not currently listed on Import Alert 16-124 (“Detention Without Physical Examination of Aquaculture Seafood Products Due to Unapproved Drugs”), Import Alert 16-127 (“Detention Without Physical Examination of Crustaceans Due to Chloramphenicol”), or Import Alert 16-129 (“Detention Without Physical Examination of Seafood Products Due to Nitrofurans”), had one entry line refused for breaded shrimp contaminated with veterinary drug residues by the Division of West Coast Imports on December 30, 2020.
In addition to these shipments, the FDA reported refusing another seven entry lines of shrimp from India, Indonesia, the Philippines, and Thailand for the presence of salmonella. Combined, refusals of shrimp entry lines for filth, listeria, salmonella, and veterinary drug residues accounted for a significant majority of all of the 194 seafood entry lines refused by the FDA in November and December.
