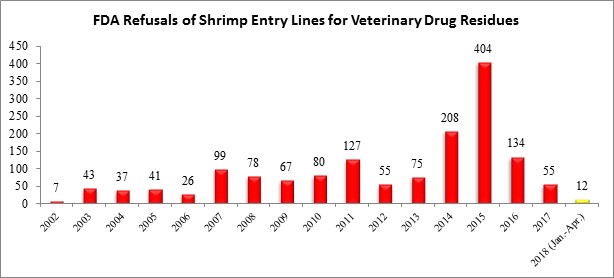
| The three entry line refusals in April were each from different countries:
· Llaos Acuacultura SA de CV (Mexico), a company that was added to Import Alert 16-124 for enrofloxacin in its shrimp on April 25, 2018, had one entry line refused for shrimp contaminated with veterinary drug residues by the Division of Southwest Imports on April 3, 2018 based on the FDA’s own sample analysis;
· M/S. Milesh Marine Exports (India), a company that was added to Import Alert 16-124 for the detection of AOZ (nitrofurans) in its shrimp on April 20, 2018, had one entry line refused for shrimp contaminated with veterinary drug residues by the Division of West Coast Imports on April 5, 2018 based on the FDA’s own analysis; and
· Rao Ping Le Ja Seafood Development (China) , a company that has not been exempted from Import Alert 16-131 (“Detention Without Physical Examination of Aquacultured Shrimp, Dace, and Eel from China – Presence of New Animal Drugs and/or Unsafe Food Additives”), had one entry line refused for shrimp contaminated with veterinary drug residues by the Division of West Coast Imports on April 11, 2018.
Also in April, the FDA reported refusing an additional twelve entry lines of seafood from China (ten of eel and two of tilapia) for veterinary drug residue contamination.
Further, the FDA also reported refusing another ten (10) shrimp entry lines because of salmonella in April, with all of these shipments originating in India. Through the first four months of this year, the FDA has already refused 46 entry lines of shrimp for salmonella – virtually all originating from India. These refusals have been made at multiple ports throughout the country, encompassing the FDA’s Divisions of West Coast, Northeast, and Southeast Imports.
|