Yesterday, the U.S. Food and Drug Administration (FDA) reported that there were just 59 total seafood entry line refusals in November, of which none were of shrimp for reasons related to banned antibiotics. No seafood entry line refusals of any kind were reported after November 22nd, indicating that the FDA’s reporting likely only covers the first three weeks of the month.
Further, the FDA reported an additional 19 seafood entry line refusals in October, of which one (5.3%) was of shrimp for reasons related to banned antibiotics. With this additional shipment, the FDA has now refused a total of 61 entry lines of shrimp for reasons related to veterinary drug residues in 2019.
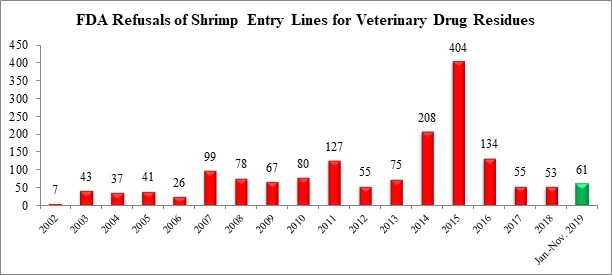
The one additional shrimp entry line refused in October for veterinary drug residues was from Mexico:
- Negocio Agricola San Enrique S.A. de CV (Mexico), a company that is not currently listed on Import Alert 16-124 (“Detention Without Physical Examination of Aquaculture Seafood Products Due to Unapproved Drugs”), Import Alert 16-127 (“Detention Without Physical Examination of Crustaceans Due to Chloramphenicol”), or Import Alert 16-129 (“Detention Without Physical Examination of Seafood Products Due to Nitrofurans”), had one entry line refused for shrimp contaminated with veterinary drug residues by the Division of Southwest Imports on October 29, 2019.
Additionally, the FDA reported refusing another seven entry lines of shrimp because of the presence of salmonella: five from India (Milesh Marine Exports Pvt. Ltd.) by the Division of Northeast Imports; one from Sri Lanka (Taprobane Seafoods (Pvt.) Ltd.) by the Division of Northeast Imports; and one from the Philippines (HJR International Corporation) by the Division of West Coast Imports.
