Responding to concerns expressed by the Southern Shrimp Alliance regarding the contamination of Vietnamese seafood exports to the U.S. market, the U.S. Food and Drug Administration (FDA) reported that following organizational changes in the Ministry of Fisheries, the Vietnamese government did not extend or revoked:
a number of regulatory Directives and Decisions that are legally enforceable in Vietnam, including Ministry of Fisheries Decision No. 29/2005/QD-BTS (November 1, 2005) that required all consignments of basa, tra, shrimp and crabmeat to be tested before shipment to the U.S. . . .
The letter does not state when Vietnam unilaterally walked away from its commitment to test consignments. However, it appears likely that Vietnamese authorities were no longer testing shrimp exported to the United States in 2011 at the same time as sharp increases in the detection of banned substances in Vietnamese shrimp were being reported by the Japanese and Canadian governments.
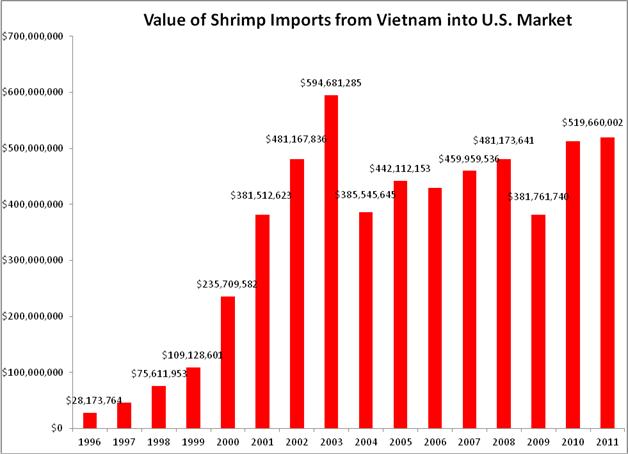
With increased scrutiny of their exports in other major seafood importing markets and in the absence of the commitment to test shrimp destined for the U.S. market, despite massive disease outbreaks in Vietnamese shrimp aquaculture that limited production, the value of Vietnamese shrimp exports to the U.S. in 2011 (nearly $520 million) was the highest seen since 2003; the second highest on record:
The unilateral decision by the Vietnamese government to drop its commitment to test shrimp exports to the U.S. was not widely publicized. Because the move corresponds with recent findings of widespread use of banned antibiotics and herbicides in the country’s aquaculture, the change in Vietnam’s approach should have sounded alarms in the context of current negotiations for a Trans-Pacific Partnership (TPP) Free Trade Agreement that would include, among other countries, Vietnam and the United States.
In comments filed last Thursday with the House Ways and Means Committee regarding the future of U.S. trade negotiations, SSA emphasized the deterioration of the Vietnamese seafood export aquaculture industry and argued that “a continuing commitment to ensuring the health and safety of American consumers must be a baseline prerequisite for further TPP discussions.” There should be no dispute that the food safety obligations and commitments of a trading partner be allowed to worsen in the context of a free trade agreement.
As for the future, the FDA’s letter reported that after inspecting thirteen Vietnamese seafood processors over the last two years, the agency planned on inspecting twenty-eight Vietnamese seafood processors in fiscal year 2012. The FDA also expressed an intention to send a team of aquaculture experts to Vietnam in May 2012 to “assess current programs and strategies the country’s government and industry implement and their ability to institute adequate food safety measures to control animal drug and chemical residues in aquaculture products intended for the U.S. market.”
Read the FDA’s Letter to the Southern Shrimp Alliance here:
https://shrimpalliance.com/?p=1756
Read the Southern Shrimp Alliance’s Written Statement to the House Ways and Means Committee here:
https://shrimpalliance.com/?p=1762
News
FDA: Vietnam Walked Away from Commitment to Test Shrimp, Basa, Tra, and Crabmeat Exports to the United States
Responding to concerns expressed by the Southern Shrimp Alliance regarding the contamination of Vietnamese seafood exports to the U.S. market, the U.S. Food and Drug Administration (FDA) reported that following organizational changes in the Ministry of Fisheries, the Vietnamese government did not extend or revoked:
a number of regulatory Directives and Decisions that are legally enforceable in Vietnam, including Ministry of Fisheries Decision No. 29/2005/QD-BTS (November 1, 2005) that required all consignments of basa, tra, shrimp and crabmeat to be tested before shipment to the U.S. . . .
The letter does not state when Vietnam unilaterally walked away from its commitment to test consignments. However, it appears likely that Vietnamese authorities were no longer testing shrimp exported to the United States in 2011 at the same time as sharp increases in the detection of banned substances in Vietnamese shrimp were being reported by the Japanese and Canadian governments.
With increased scrutiny of their exports in other major seafood importing markets and in the absence of the commitment to test shrimp destined for the U.S. market, despite massive disease outbreaks in Vietnamese shrimp aquaculture that limited production, the value of Vietnamese shrimp exports to the U.S. in 2011 (nearly $520 million) was the highest seen since 2003; the second highest on record:
The unilateral decision by the Vietnamese government to drop its commitment to test shrimp exports to the U.S. was not widely publicized. Because the move corresponds with recent findings of widespread use of banned antibiotics and herbicides in the country’s aquaculture, the change in Vietnam’s approach should have sounded alarms in the context of current negotiations for a Trans-Pacific Partnership (TPP) Free Trade Agreement that would include, among other countries, Vietnam and the United States.
In comments filed last Thursday with the House Ways and Means Committee regarding the future of U.S. trade negotiations, SSA emphasized the deterioration of the Vietnamese seafood export aquaculture industry and argued that “a continuing commitment to ensuring the health and safety of American consumers must be a baseline prerequisite for further TPP discussions.” There should be no dispute that the food safety obligations and commitments of a trading partner be allowed to worsen in the context of a free trade agreement.
As for the future, the FDA’s letter reported that after inspecting thirteen Vietnamese seafood processors over the last two years, the agency planned on inspecting twenty-eight Vietnamese seafood processors in fiscal year 2012. The FDA also expressed an intention to send a team of aquaculture experts to Vietnam in May 2012 to “assess current programs and strategies the country’s government and industry implement and their ability to institute adequate food safety measures to control animal drug and chemical residues in aquaculture products intended for the U.S. market.”
Read the FDA’s Letter to the Southern Shrimp Alliance here:
https://shrimpalliance.com/?p=1756
Read the Southern Shrimp Alliance’s Written Statement to the House Ways and Means Committee here:
https://shrimpalliance.com/?p=1762
Share This Article
Join the Mailing List
Get news from Southern Shrimp Alliance straight to your inbox!
By submitting this form, you are consenting to receive marketing emails from: . You can revoke your consent to receive emails at any time by using the SafeUnsubscribe® link, found at the bottom of every email. Emails are serviced by Constant Contact
Related Posts
First Quarter Report
Southern Shrimp Alliance Applauds Introduction of the Save Our Shrimpers Act
Antibiotic-Contaminated Shrimp from Two More BAP-Certified Exporters Refused by FDA in March
Southern Shrimp Alliance Commends Rep. Graves and Rep. Peltola for Demanding Action on Indian Shrimp
SSA Asks Labor Department to Add Indian Shrimp to Forced/Child Labor Lists
Senator Cassidy Demands Protections for Whistleblower and Investigation of Claims