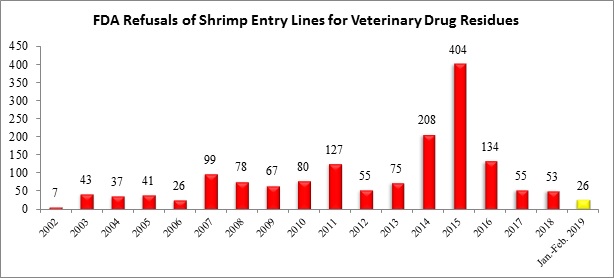
The U.S. Food and Drug Administration (FDA) today released information regarding the seventy-four (74) entry lines of seafood refused in February. After reporting the refusal of twenty-six (26) entry lines of shrimp for reasons related to banned antibiotics in January, FDA reported no such refusals last month.
In fact, just four (4) entry lines of shrimp were refused for any reason last month. Three (3) shrimp entry lines from the Venezuelan exporter Comercializadora Lugo, C.A. were refused because of issues with the labeling of this shrimp and one (1) shrimp entry line from the Indian exporter B-One Business House Private Limited was refused because of filth and the potential presence of salmonella in the shrimp. Through the first two months of this year, the FDA has now reported refusing seven (7) entry lines of Indian shrimp for reasons related to salmonella.
The table below summarizes shrimp entry line refusals for reasons related to veterinary drug residues from 2002 through February 2019: