Last week, the U.S. Food and Drug Administration (FDA) published detailed data regarding 74 seafood entry line refusals in December, of which four (5.4%) were for shrimp for reasons related to banned antibiotics and an additional fifteen seafood entry line refusals in November, of which five (33.3%) were for shrimp for reasons related to banned antibiotics.
With its reporting complete for the year, the FDA refused a total of 59 entry lines of shrimp for banned antibiotics in 2023 – one less than it had in 2022.
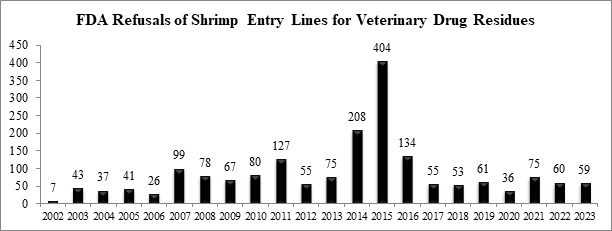
The nine newly-reported entry lines of shrimp refused for banned antibiotics in November and December were for shipments from two Indian shrimp exporters, an Ecuadorian shrimp exporter, and a Thai shrimp exporter:
- Calcutta Seafoods Pvt. Ltd. (India), a company that currently operates under a four-star Best Aquaculture Practices (BAP) certification for its processing plant (P10567), with additional BAP certifications for multiple shrimp farms, and that was added to Import Alert 16-129 (“Detention Without Physical Examination of Seafood Products Due to Nitrofurans”) for its shipments of shrimp on July 19, 2023, had four entry lines refused for shrimp contaminated with nitrofurans by the Division of West Coast Imports on November 29, 2023;
- S.A. Exports (India), a company that currently operates under BAP certifications for two shrimp farms (F12601 and F12604), and that was added to Import Alert 16-129 (“Detention Without Physical Examination of Seafood Products Due to Nitrofurans”) for its shipments of shrimp on December 13, 2023, had one entry line refused for shrimp contaminated with nitrofurans by the Division of Southeast Imports on December 19, 2023;
- COFIMAR SA (Ecuador), a company that currently operates under a four-star BAP certification for its processing plant (P10744), with an additional BAP certification for a shrimp farm (F12708), and that was added to Import Alert 16-124 (“Detention Without Physical Examination of Aquaculture Seafood Products Due to Unapproved Drugs”) for gentian violet in its shipments of shrimp on December 27, 2023, had one entry line refused for shrimp contaminated with veterinary drug residues by the Division of West Coast Imports on November 29, 2023;
- Okeanos Food (Thailand), a company that currently operates under a four-star Best Aquaculture Practices certification for its processing plant (P10123) with additional BAP certifications for a hatchery and multiple shrimp farms, and that was added to Import Alert 16-129 (“Detention Without Physical Examination of Seafood Products Due to Nitrofurans”) for its shipments of shrimp, breaded shrimp, and shrimp in various food preparations (dumplings, chowder, soup, pasta, etc.) on November 27, 2023, had three entry lines refused for shrimp contaminated with nitrofurans and veterinary drug residues by the Division of Northeast Imports on December 27, 2023.
In addition, on December 15, 2023 the FDA also added another processing plant operating under a four-star Best Aquaculture Practices certification, Kader Exports Private Limited Unit 04 (P10302) to Import Alert 16-124 (“Detention Without Physical Examination of Aquaculture Seafood Products Due to Unapproved Drugs”) for leucomalachite green in its shipments of shrimp.
