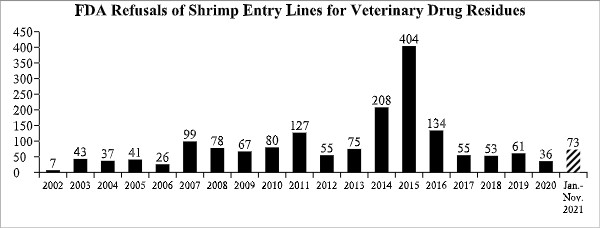
The U.S. Food and Drug Administration (FDA) published detailed data over the weekend regarding 54 total seafood entry line refusals in November, of which 2 (3.7%) were of shrimp for reasons related to banned antibiotics. The FDA also reported an additional 29 seafood entry line refusals in October that were not part of the agency’s reporting last month. None of these additional reported entry line refusals involved shipments of shrimp.
With one month left in 2021, the FDA has now refused a total of 73 entry lines of antibiotic-contaminated shrimp, the highest number of entry line refusals for these reasons since 2016.
The two shrimp entry lines refused in November were for shipments from Malaysia and Vietnam, as described in more detail below:
- Oceanbest (M) Sdn. Bhd. (Malaysia), a company located in peninsular Malaysia that is not on the green list of Import Alert 16-136 (“Detention Without Physical Examination of Aquacultured Shrimp and Prawns from Peninsular Malaysia Due to Presence of Drug Residues from Unapproved Animal Drugs or the Presence of Unsafe Food Additives”), had one entry line refused for shrimp contaminated with veterinary drug residues and unsafe additives by the Division of West Coast Imports on November 22, 2021; and
- Can Tho Import Export Fishery Limited Company, aka CAFISH (Vietnam), a company that is currently listed on Import Alert 16-129 (“Detention Without Physical Examination of Seafood Products Due to Nitrofurans”) as of March 4, 2021, had one entry line refused for shrimp contaminated with nitrofurans by the Division of Southeast Imports on November 15, 2021.
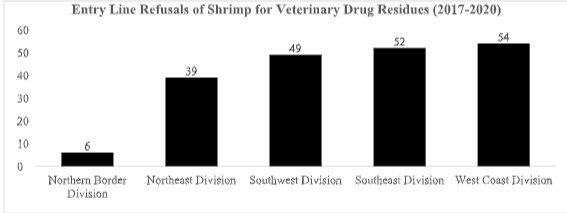
The 73 refusals of shrimp entry lines for reasons related to veterinary drug residues in 2021 have all been from just three divisions of the FDA – the West Coast, Northeast, and Southeast-as shown below:
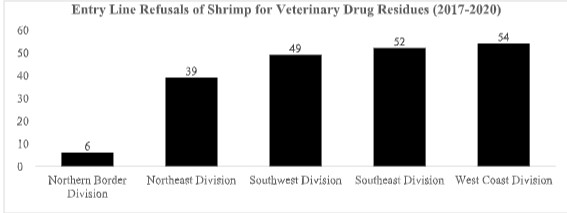
The concentration of refusals in these three divisions, and the complete absence of any such entry line refusals from the Division of Southwest Imports, sharply contrasts with the FDA’s actions in the previous four years. As shown below, between 2017 and 2020, the FDA’s Division of Southwest Imports was the third largest source of shrimp entry line refusals for reasons related to banned antibiotics, while the Division of Southeast Imports was the second largest source. This year, however, such entry line refusals from both southern divisions of the FDA appear to have collapsed: