Over the weekend, the U.S. Food and Drug Administration (FDA) released information regarding import refusals in May indicating that 8 out of the 111 (7.2%) total seafood entry line refusals last month were of shrimp for reasons related to banned antibiotics. This is the highest total of refusals of entry lines for these reasons since April 2017.
For the year, the FDA has now reported refusing a total of 20 entry lines of shrimp for reasons related to veterinary drug residues:
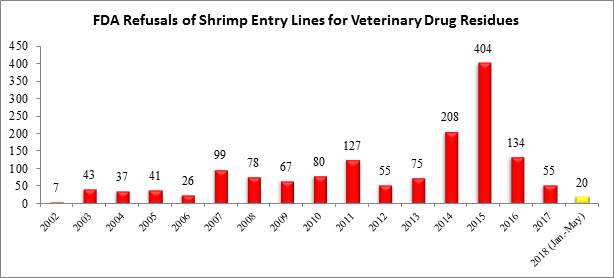
The eight entry line refusals in May were from five different countries and involved four different FDA regional districts:
- Zhanjiang Longwei Aquatic Products Industry Co., Ltd. (China), a company that has been exempted from Import Alert 16-131 (“Detention Without Physical Examination of Aquacultured Shrimp, Dace, and Eel from China – Presence of New Animal Drugs and/or Unsafe Food Additives”) since June 2013, had two entry lines refused for breaded shrimp contaminated with veterinary drug residues by the Division of West Coast Imports on May 21, 2018;
- Savvy Seafood Inc. (China), a company that is no longer exempted from Import Alert 16-131 (“Detention Without Physical Examination of Aquacultured Shrimp, Dace, and Eel from China – Presence of New Animal Drugs and/or Unsafe Food Additives”), had one entry line refused for breaded shrimp contaminated with nitrofurans by the Division of West Coast Imports on May 17, 2018;
- Lee Fung Marine Products Trading Co (Hong Kong), a company that is not currently listed on Import Alerts 16-124 (unapproved drugs), 16-127 (chloramphenicol), or 16-129 (nitrofurans), had one entry line refused for shrimp contaminated with veterinary drug residues by the Division of East Coast Imports on May 1, 2018;
- Edhayam Frozen Foods Pvt. Ltd. (India), a company that is not currently listed on Import Alerts 16-124, 16-127, or 16-129, had one entry line refused for shrimp contaminated with veterinary drug residues and nitrofurans by the Division of Southwest Imports on May 21, 2018;
- Freshly Frozen Foods (United Arab Emirates), a company that is not currently listed on Import Alert 16-124, 16-127, or 16-129, had two entry lines refused for shrimp contaminated with veterinary drug residues and nitrofurans by the Division of Southwest Imports on May 10, 2018; and,
- Winful Seafood International Ltd. (Canada), a company that is not currently listed on Import Alert 16-124, 16-127, or 16-129, had one entry line refused for shrimp contaminated with nitrofurans by the Division of Northern Border Imports on May 18, 2018.
For the first time this year, the FDA did not also report refusing shrimp for reasons related to salmonella. Prior to May, the agency had reported refusing 46 entry lines of shrimp for salmonella in 2018 – virtually all originating from India.
