This past holiday weekend, the U.S. Food and Drug Administration (FDA) published detailed data regarding 63 total seafood entry line refusals in June, of which nine (14.3%) were of shrimp for reasons related to banned antibiotics. In addition to these nine entry lines, another entry line of shrimp was refused for the presence of pesticides, while another five entry lines of shrimp were refused either for the presence of salmonella or being filthy (or both).
Over the first half of the year, the FDA has refused a total of 45 entry lines of shrimp for reasons related to banned antibiotics and is on track to refuse the largest number of entry lines of shrimp for veterinary drug residues since 2016.
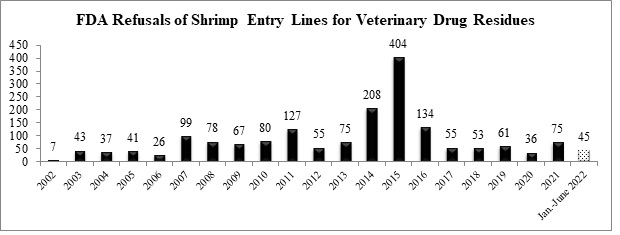
The nine shrimp entry lines refused for antibiotic residues in June were for shipments from two different companies in India and a company in Bangladesh:
- BD Seafood Limited (Bangladesh), a company that is currently listed on Import Alert 16-129 (“Detention Without Physical Examination of Seafood Products Due to Nitrofurans”) as of May 16, 2022, had three entry lines refused for shrimp contaminated with nitrofurans and veterinary drug residues by the Division of West Coast Imports on June 3, 2022;
- Coastal Corporation Ltd. (India), a company that is currently listed on Import Alert 16-129 (“Detention Without Physical Examination of Seafood Products Due to Nitrofurans”) as of June 23, 2022, had one entry line refused for shrimp contaminated with veterinary drug residues by the Division of Northern Border Imports on June 7, 2022; and
- Kader Exports Private Limited, Unit 5 (India), a company that is currently listed on Import Alert 16-124 (“Detention Without Physical Examination of Aquaculture Seafood Products Due to Unapproved Drugs”) for leucomalachite green as of May 31, 2022, had five entry lines refused for shrimp contaminated with veterinary drug residues by the Division of West Coast Imports on June 17, 2022.
In addition to these nine shipments, the Division of West Coast Imports of the FDA also refused a shipment of shrimp from the Vietnamese exporter Tra Kha Seafood Processing Factory (F69) due to the presence of a pesticide, while another five (5) entry lines of shrimp from India and Ecuador were refused for the presence of salmonella or for being filthy (or both), including:
- One entry line of shrimp from Sterling Foods in India was refused on June 8, 2022 by the Division of Northeast Imports for being filthy and for the presence of salmonella;
- A total of three entry lines of shrimp manufactured by Industrial Pesquera Santa Priscila S.A. (Factory 2) in Ecuador was refused on June 7, 2022 by the Division of Southwest Imports for the presence of salmonella; and
- One entry line of shrimp from Suryamitra Exim PVT Ltd. in India was refused on June 2, 2022 by the Division of Southeast Imports for being filthy and for packaging that did not provide the name and place of business of the manufacturer, packer, or distributor.
