For the month of March, the U.S. Food and Drug Administration (FDA) reports that 5 out of the 171 (2.9%) total seafood entry line refusals were of shrimp for reasons related to banned antibiotics. In addition, the FDA reported refusing an entry line of shrimp from Vietnam because of the presence of pesticides, as well as six entry lines of shrimp from India because of the presence of salmonella.
The table below summarizes shrimp entry line refusals for reasons related to veterinary drug residues from 2002 through March 2019.
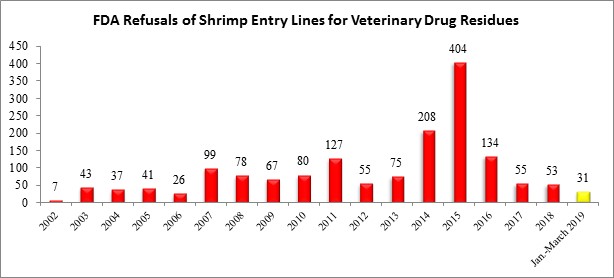
All five shrimp entry line refusals in March for veterinary drug residues were from the same exporter from Vietnam that had multiple entry lines refused for the same reasons in November:
- Minh Phu – Hau Giang Seafood Joint Stock Company (Vietnam), a company that is has been listed on Import Alert 16-124 (“Detention Without Physical Examination of Aquaculture Seafood Products Due to Unapproved Drugs”) since January 23, 2019 for ciprofloxacin in its shrimp shipments, had five entry lines refused for shrimp contaminated with veterinary drug residues by the Division of West Coast Imports on March 19, 2019.
In addition, the Division of Southwest Imports refused an entry line of shrimp shipped by NTSF Seafoods Joint Stock Company because of the presence of a pesticide chemical residue. Shrimp shipments are rarely refused for reasons related to the presence of pesticides and no companies are currently listed on Import Alert 99-08 (“Detention Without Physical Examination of Processed Human and Animal Foods for Pesticides”) for shrimp.
Finally, another six entry lines of shrimp from India, from three different companies, were refused because of salmonella last month.
